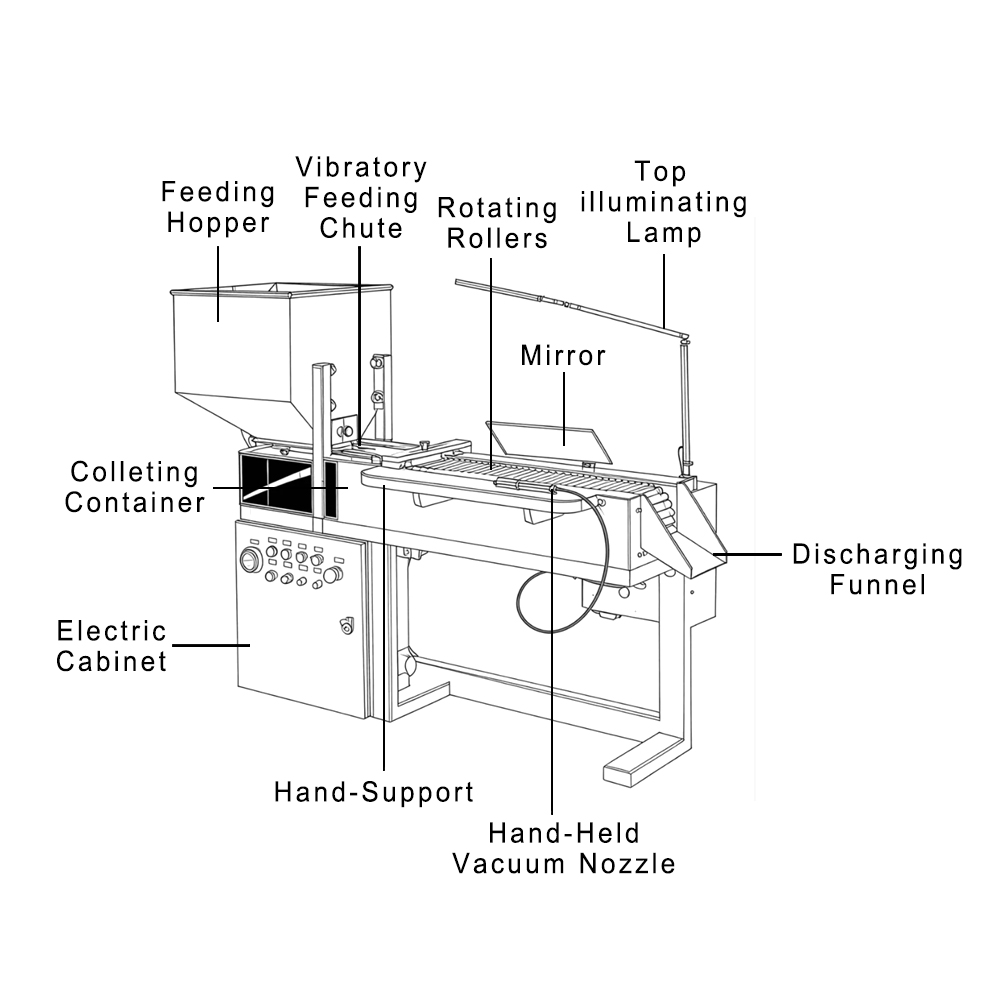
When you manufacture a drug, every detail matters. This is also where two steps are often confused: qualification and validation. They may sound alike, but they serve very different purposes.

Mixing them up can result in product failures, citations, or even worse consequences.
In fact, during a routine GMP audit, inspectors flag “major” deficiencies in over 37% of cases, many of which are tied back to inadequate validation or qualification practices. Now you see how high the stakes are.
So, let’s clear the fog and take you through the differences between qualification and validation.
Key Takeaways: Difference Between Qualification and Validation
- Scope Matters: Qualification applies to equipment, facilities, and utilities, while validation deals with processes, analytical methods, computerized systems, and final products.
- Different Goals: The end goal for qualification is whether tools are suitable for their purpose, while for validation, it’s that the processes work consistently.
- Regulatory Angle: Validation directly guarantees product effectiveness; meanwhile, qualification sets the stage for GMP adherence.
- When It’s Done: Companies need qualification during the setup, modification, or equipment. On the other hand, validation is carried out whenever methods are developed or altered.
What Is Qualification?
Qualification is the documented process of proving that equipment, systems, and utilities used in pharmaceutical manufacturing are installed and working as intended.

It ensures that machines, facilities, and supporting utilities, such as HVAC, water, or compressed air systems, function as intended. The goal is to confirm they can consistently perform according to predefined standards and GMP requirements.
Stages of Qualification
Qualification is a series of stages that gradually build confidence in equipment performance. These stages include:
- URS (User Requirement Specification): It outlines the exact requirements the equipment must fulfill, from process performance to regulatory alignment.
- Risk Management (RM): The process identifies potential failures in advance and puts safeguards in place to protect product quality.
- Validation Master Plan (VMP): A high-level document that lays out the overall strategy, responsibilities, and schedule for all validation tasks.
- FAT and SAT (Factory and Site Acceptance Tests): Equipment is first tested at the supplier’s site. Then, they are re-tested after installation at the production facility to confirm full compliance.
- DQ, IQ, OQ, PQ (Qualification Phases): Together, these steps verify the design, check correct installation, and test functionality under defined ranges. They prove consistent performance under real manufacturing conditions.
What Is Validation?
Validation extends beyond individual equipment and encompasses the entire process, method, or system. It is the documented evidence that a process consistently produces a product that meets its intended quality requirements.

Unlike qualification, validation ensures the end product itself is reliable. This covers manufacturing processes, cleaning procedures, analytical methods, and computerized systems used in pharma operations.
Types of Validation
Pharmaceutical manufacturing requires different forms of validation, depending on the scope:
- Process Validation: It’s about proving, through evidence, that every manufacturing run consistently produces tablets that meet set specifications.
- Cleaning Validation: By verifying that no harmful residue is left behind after cleaning, it protects against cross-contamination from one batch to the next.
- Method Validation: Analytical test methods only hold value if they are accurate, so this step confirms their reliability before being applied in real testing.
- Computer System Validation (CSV): In modern pharma, CSV ensures these tools operate correctly and in compliance with regulations.
Key Differences Between Qualification and Validation
Pharmaceutical manufacturing is highly regulated because small mistakes can affect patient safety. This is why both qualification and validation exist.
To clarify the differences between qualification and validation, here’s a detailed examination of how they are applied in practice.
1. Focus Area

Qualification is all about equipment, utilities, and facilities. For instance, a water purification system, HVAC unit, or tablet press machine must be checked to prove it works exactly as intended.
Validation, however, is broader. It examines the processes and methods that utilize those machines. An example of it is how granulation, coating, or cleaning is carried out, and whether the same quality results are achieved every single time.
2. Objective
The main goal of qualification is to ensure that the equipment or system is fit for purpose before it’s used in production. Remember, a machine cannot be trusted until it has been proven to work correctly under GMP requirements.
On the other hand, validation aims to ensure that the final product and process are consistently safe and compliant. A validated process shows regulators and patients that, regardless of the number of batches produced, they will all meet the same high-quality standards.
3. Regulatory Role
If you think of a foundation for compliance, qualification comes to mind first. Regulators, such as the FDA or EMA, expect pharmaceutical companies to demonstrate that every piece of equipment is reliable. Without qualification, there’s no credibility in any further testing.
Validation is the next layer that regulators examine because it proves the entire manufacturing process will not compromise safety or efficacy. In fact, an application can be rejected if process validation is incomplete.
4. Timing of Application
Validation is performed whenever a new process, method, or system is developed or significant changes are made. When you change the way the tablets are coated, you must get the process validated to prove it has the same coating quality.
Alternatively, qualification is required whenever new equipment, utilities, or facilities are installed or undergo major changes. For instance, if a company buys a new blister packaging machine, it must be qualified before it can be used.
5. Dependency
Validation and qualification are interlinked, but qualification always comes first. You cannot validate a process using equipment that hasn’t been qualified, because the results won’t be trustworthy.
Qualification ensures that the equipment is reliable, and validation builds upon it to confirm that the final product meets all quality expectations.
6. Impact on Products
Qualification has an indirect impact on product quality, while validation has a direct effect. By proving machines and systems work as intended, qualification reduces the risk of failures during production. However, it doesn’t guarantee product quality on its own.
Validation confirms that the process consistently delivers safe and high-quality medicines. This is why validation is often seen as the ultimate safeguard for patients.
Why Qualification + Validation Are Important for Pharma Companies
You can have the most advanced machines, but without rigorous qualification and validation, your entire operation is vulnerable.
Below are several reasons why both are non-negotiable.
1. Protecting Product Quality

If equipment or systems aren’t qualified, you risk hidden malfunctions. Meanwhile, if processes aren’t validated, you can end up with batches that fail strength, dissolution, or purity tests, leading to failure.
In fact, regulators now frequently cite process validation deficiencies in warning letters. For example, in FY 2021, the FDA criticized seven companies for inadequate process validation practices.
2. Avoiding Regulatory Citations and Recalls
Qualification and validation lapses are a top cause of GMP audit findings. The FDA itself recognizes that Q&V failures are “a major source of GMP deficiencies.”
When inspectors observe poor validation or missing qualification evidence, they issue warning letters or even halt your operations. That’s direct financial loss and damage to reputation.
3. Consistency and Reliability Over Time
Validation is not just about proving a process works once; it’s about ongoing confidence that each batch will be as good as the last. Qualification ensures the backbone (equipment, utilities) remains solid for that consistency.
By combining both, you establish a state of control where variations are detected early and corrected before they impact product quality.
FAQs
1. What are the common problems if validation is skipped?
Skipping validation can lead to inconsistent batches, product failures in quality tests, or even safety risks for patients. It often results in costly recalls, regulatory warnings, or shutdowns. For instance, if a sterilization process isn’t validated, you can’t prove every batch is free from harmful microbes.
2. Who is responsible for qualification and validation in a company?
Typically, the Quality Assurance (QA) department oversees qualification and validation. However, engineering, production, and regulatory teams also play key roles. QA ensures proper documentation, while engineering runs tests and production teams operate the equipment.
3. What documents are required for qualification and validation?
Essential documents include protocols, reports, test results, and approvals. For qualification, this means DQ, IQ, OQ, and PQ documents. On the other hand, validation includes process validation protocols, cleaning validation reports, and continued process verification data.
Keep Risk From Disrupting Your Production
Every year, pharma companies face costly recalls, FDA warning letters, and damaged reputations. It all happens because companies overlook the difference between qualification and validation. These are compliance gaps that can shut down your production lines.
You can avoid them simply by investing in proper qualification and validation processes. Using high-quality GMP-compliant machines can further support these efforts.
Fortunately, at Finetech, we do precisely that. We help companies meet regulatory expectations, eliminate operational risks, and maintain the highest quality standards.
Get a free quote today and get the machine you’ve had your eyes on for quite a while!